Research Pipeline
- Discovery
- Lead optimization
- Pre-clinical
- Phase Ⅰ
- Phase II
- Phase III
- Registration
| Category | Code name | Development phase | Being in adjustment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NBE | CreaVax-HCC | Liver cancer (for surgical patients) | |||||||
| CreaVax-HCC | Liver cancer (targeted at carotid chemistry and color transmutation patients) | ||||||||
CreaVax-HCC
Summary of Phase II clinical trial
- CreaVax-HCC was well tolerated to firstly diagnosed early HCC patients.
- CreaVax-HCC showed significant inhibition of tumor recurrence with curative hepatic resection suggesting increase of recurrence free survival in two years
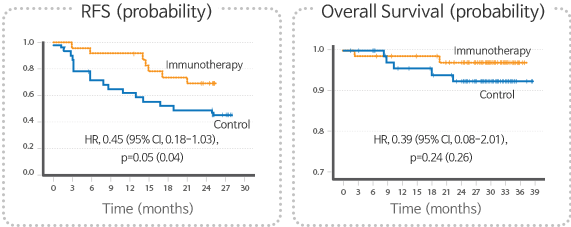
Summary of Phase III clinical trial
- Study design : A Multi-centered, randomized, double-blind, placebo-controlled Phase III study on the efficacy and safety of CreaVax-HCC in patients with hepatocellular carcinoma for comparing after curative hepatic resection
- Target patients : Early HCC patients after curative hepatic resection with CR confirmation (n=166)
- Current state : Completion of Enrollment